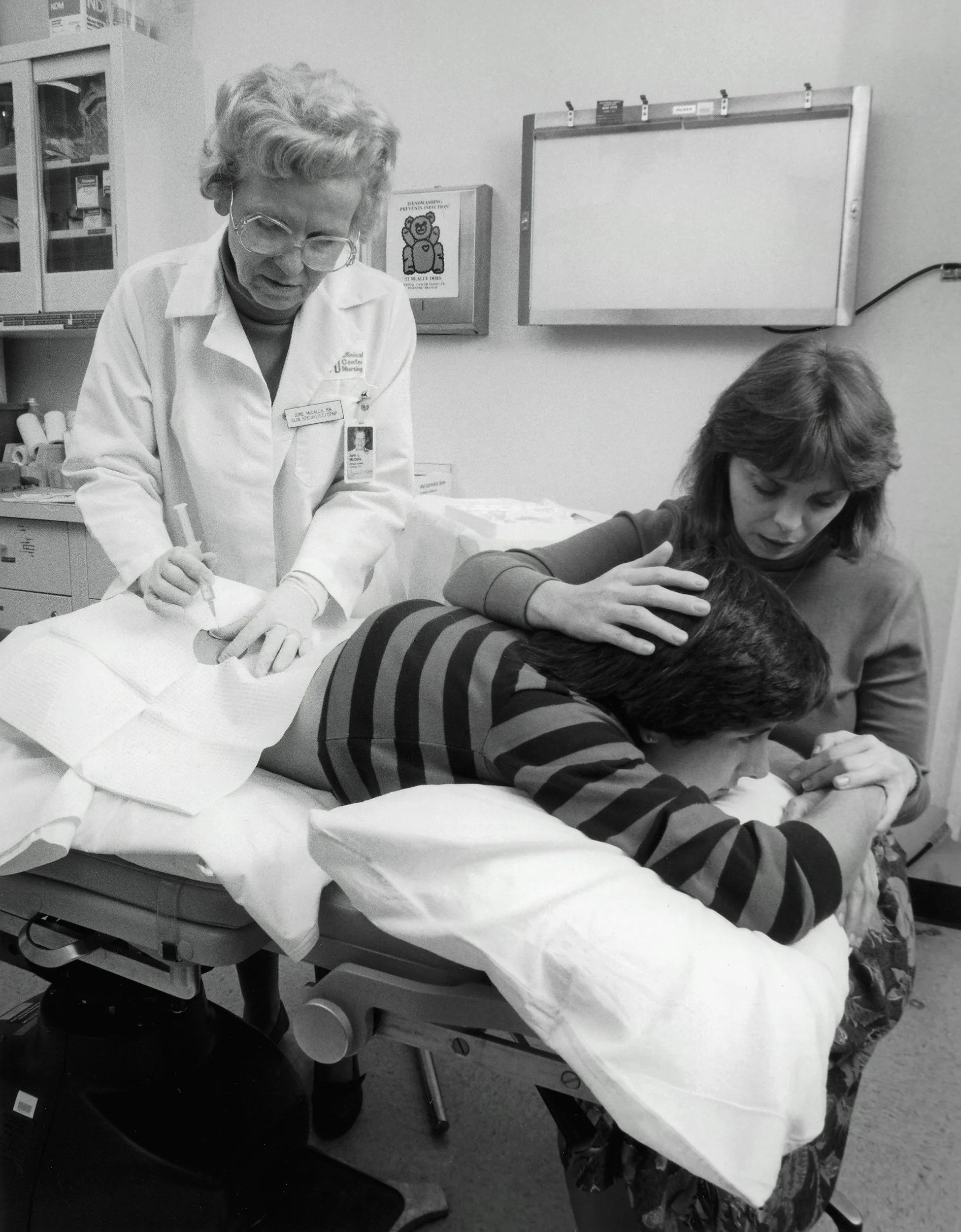
From Hiroshima to healing. How bone marrow transplantation progressed from early trials to safe, accredited therapy, with UK pioneers, EBMT standards, and Biovault’s accredited processing.
Cellular Therapies, Innovation
Sarah Conway
Explore how PBSCs enabled CAR-T and immune effector cell therapies, and how Biovault supports safe processing, storage and delivery of advanced cellular medicines.
Cellular Therapies
Sarah Conway
How peripheral blood stem cells became the preferred source for transplantation, and how Biovault supports PBSC collection pathways through compliant processing and storage.

Innovation
Sarah Conway
How Biovault Technical engineered new cryoshipper racks and CRF freezer racks to enhance safety, usability and compliance for cellular therapies.

Sustainability, Innovation, Ethical Practices
Sarah Conway
As the UK’s ethical bioresource, Biovault is leading the way in slashing processing and storage emissions as part of its pledge to achieve Net Zero.