From Transplant to Targeted Therapy
Welcome to Part 3 of our The Evolution of Cell Therapy series. In Part 1, we saw how bone marrow transplantation proved that cells themselves could be used as medicine. In Part 2, we explored how peripheral blood stem cells (PBSCs) made that idea practical at scale, improving donor experience and embedding reliable systems for collection, processing and storage.
In this final article, we consider how immune effector cell (IEC) therapies, including CAR-T, represent the next phase of this evolution. IECs are often described as revolutionary. In practice, they are also a continuation, growing directly from the scientific, clinical, and operational foundations established by transplantation and PBSC practice.
We explore the different types of IECs, their place within today’s therapeutic ecosystem, and their emerging potential beyond cancer. We also highlight the critical role of standards-led infrastructure in enabling the safe delivery of personalised, cell-based therapies.
How did immune effector cell therapies evolve from bone marrow transplantation?
A bone marrow tap - National Cancer Institute (NCI)
Haematopoietic transplantation established a critical principle: the immune system can be rebuilt, reset and redirected using carefully handled cells. Over decades, this led to validated apheresis pathways, controlled laboratory processing, cryopreservation methods that preserve cell function, and rigorous systems for traceability and release.
Immune effector cell therapies rely on exactly the same foundations. As treatments have become more personalised, the importance of these systems has increased rather than diminished. IEC therapies did not replace transplantation. They grew out of it.
What are immune effector cell therapies, such as CAR-T?
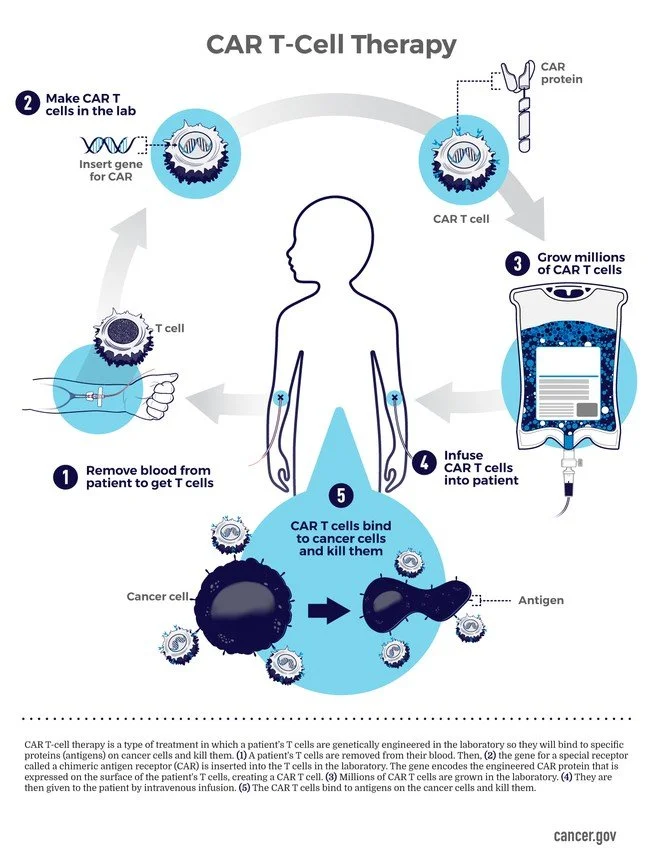
Immune effector cell therapies are treatments in which immune cells are the active therapeutic agent. These cells are typically collected from a patient, modified or expanded outside the body, and returned to deliver a targeted immune response.
CAR-T therapy is one example within this wider group. In CAR-T, a patient’s T cells are collected from the blood and reprogrammed in the laboratory to recognise a specific target, most often on cancer cells. The modified cells are expanded, frozen, stored and later infused back into the patient.
Unlike conventional medicines, IEC therapies are living products. They are often patient-specific, manufactured to order and time-critical. Safe delivery depends as much on handling, identity assurance and logistics as on biological design.
What therapies sit alongside CAR-T in the immune effector cell landscape?
T Lymphocyte Colourised scanning electron micrograph of a T lymphocyte (T cell). Credit: NIAID
CAR-T is the most widely recognised IEC therapy, but it is part of a broader and rapidly developing group of advanced cellular treatments. These include:
CAR-NK therapies using engineered natural killer cells
TCR-T therapies targeting intracellular antigens
Tumour-infiltrating lymphocyte (TIL) therapies expanded from tumour tissue
Dendritic cell vaccines designed to prime and direct immune responses
Gene-modified products derived from PBSC starting material
Although these therapies differ biologically, they share common operational needs. All require controlled processing, validated cryopreservation, secure storage and carefully managed release to the clinic.
Why do PBSCs remain central to modern cell therapy pathways?
PBSCs continue to play a pivotal role in cellular therapy. They are used directly as therapeutic grafts and indirectly as starting material for immune effector cell manufacture and research.
The quality of mobilisation, collection and early handling has a direct impact on downstream processing and clinical timelines. Variability at the start of the pathway can affect manufacturability, scheduling and patient readiness.
This is why PBSC expertise remains essential as IEC therapies expand.
Do CAR-T and immune effector cell therapies replace bone marrow or PBSC transplantation?
In some indications, yes. In others, no.
For certain cancers, particularly relapsed or refractory disease, CAR-T therapies are increasingly being used instead of traditional transplantation. In some settings, they are now being introduced earlier in the treatment pathway. This has led to a reduction in some types of transplant activity.
However, bone marrow and PBSC transplantation remain essential for rebuilding the blood and immune system and for treating a wide range of haematological diseases. Many IEC pathways still depend on transplant infrastructure, including apheresis services, cell processing expertise and accredited quality systems.
In practice, PBSCs and bone marrow underpin the ecosystem. Immune effector cell therapies add precision where transplantation alone is not sufficient.
Are immune effector cell therapies being explored beyond cancer, such as in multiple sclerosis?
Yes, and this is an important area of development.
Multiple sclerosis is driven by immune-mediated damage to the central nervous system. While many approved therapies broadly suppress the immune system, newer approaches aim to target specific immune cell populations or reset immune balance altogether.
Early-stage clinical trials are exploring CAR-T therapies that target disease-driving B cells in severe MS. These approaches are designed to cross the blood–brain barrier and may offer durable remission after a limited number of treatments. Alongside this, established immune reconstitution therapies, including autologous stem cell transplantation, continue to play a role in selected patients.
Although these approaches remain under investigation, they illustrate how immune effector cell strategies are beginning to extend beyond oncology.
How are immune effector cell therapies processed and cryopreserved safely?
As therapies become more personalised, the consequences of error increase.
IEC products require controlled processing environments, clear distinction between minimally manipulated and gene-modified products, and validated cryopreservation protocols. Controlled-rate freezing and vapour-phase liquid nitrogen storage are used to preserve cell viability and function.
Safe retrieval and release depend on well-rehearsed systems rather than ad-hoc handling.
Why are chain of identity and regulatory standards critical for immune effector cells?
Patient-specific therapies demand absolute confidence in identity and traceability. International standards such as ISBT 128 support unambiguous identification of cellular therapy products across organisations and systems.
In the UK, immune effector cell pathways sit within a regulated framework that includes Human Tissue Authority licensing, MHRA oversight for advanced therapy medicinal products, and FACT-JACIE standards for immune effector cell programmes.
These frameworks protect patients while enabling innovation.
How are immune effector cell therapies distributed and released to clinics?
For IEC therapies, logistics are part of clinical care.
Release and distribution must account for strict temperature control, precise timing and complete documentation. Delays or deviations can affect patient eligibility and clinical outcomes. This makes experience in cellular therapy logistics as important as transport itself.
How does Biovault support immune effector cell therapies and research?
Biovault works across transplantation, immune effector cell therapies and research. Our role includes processing, cryopreservation, storage, distribution and advisory support.
By providing standards-led infrastructure, we help organisations deliver complex cellular therapies safely while supporting the development of new treatments.
What does the future of immune effector cell therapy depend on?
Bone marrow transplantation laid the foundations. PBSCs made cellular therapy scalable.
Immune effector cell therapies add precision.
As therapies evolve, the need for robust systems does not diminish. It becomes more important. The future of advanced medicine depends not only on scientific breakthroughs, but on the infrastructure that allows living medicines to be handled, stored and delivered with confidence.
how biovault can support PBSC therapies and research
As a bioresource, our role is not limited to storage. It includes processing, problem-solving, regulatory insight, and collaboration. PBSCs sit within this wider context and continue to demonstrate how careful, standards-led practice benefits both patients and research.
References & Further Reading
Maude SL et al. Tisagenlecleucel in children and young adults with B-cell ALL. NEJM, 2018
Lee DW et al. ASTCT consensus grading for cytokine release syndrome and neurotoxicity. 2019
Locke FL et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma (ZUMA-7). NEJM, 2022
Kantoff PW et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. NEJM, 2010
Mackensen A et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nature Medicine, 2022
Passweg JR et al. Hematopoietic cell transplantation and cellular therapy activity in Europe and worldwide: the EBMT activity survey. Bone Marrow Transplantation, 2023
Yakoub-Agha I et al. Management of immune effector cell–associated toxicities: EBMT and JACIE recommendations. Bone Marrow Transplantation, 2020
Sutherland SIM et al. Dendritic cell vaccines: current status and future directions. Frontiers in Immunology, 2021
Majzner RG & Mackall CL. Clinical lessons learned from the first leg of the CAR T cell journey. Nature Medicine, 2019
Passweg JR et al. EBMT activity survey on HCT and cellular therapy.
FACT-JACIE. International Standards for Immune Effector Cell Therapy.
MHRA. Advanced therapy medicinal products: regulation and licensing.
ICCBBA. ISBT 128 standard for cellular therapy.